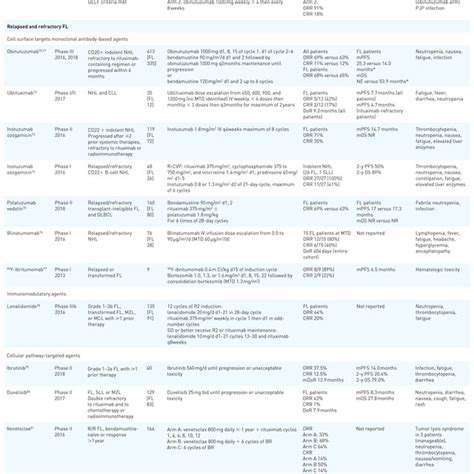
Future Directions and Clinical Trials
Harnessing CRISPR for Precision Cancer Therapy
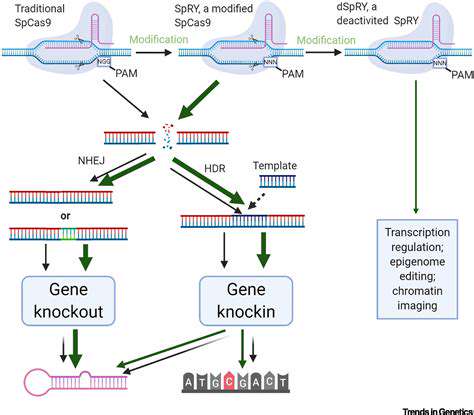
Gene editing technologies, particularly CRISPR-Cas9, are poised to revolutionize the treatment of hereditary cancers. This powerful tool offers the potential to precisely target and correct the genetic mutations that drive cancer development. Early preclinical studies demonstrate the feasibility of using CRISPR to eliminate cancer cells while sparing healthy cells, paving the way for personalized therapies tailored to individual genetic profiles. The ability to target specific genes responsible for cancer development holds immense promise for enhancing the efficacy and minimizing the side effects of current cancer treatments. This approach could significantly improve outcomes for patients with hereditary cancers, who often face aggressive and challenging disease courses.
Further development of CRISPR-based therapies requires addressing critical challenges, such as ensuring efficient delivery of the gene-editing machinery to the target cells and minimizing off-target effects. Rigorous testing and optimization of delivery methods are essential for translating these promising preclinical findings into effective clinical applications. This will likely involve exploring various delivery vectors, such as viral vectors and nanoparticles, to improve target specificity and reduce potential toxicity.
Developing Novel Diagnostic Tools
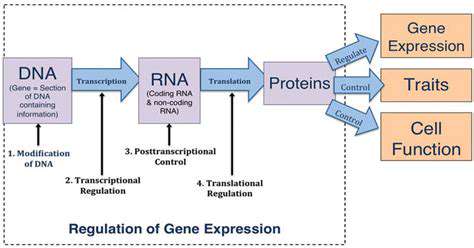
The identification of specific genetic mutations associated with hereditary cancers is critical for early detection and personalized treatment strategies. Advancements in gene sequencing technologies, coupled with gene editing tools, offer the potential to develop highly sensitive and specific diagnostic tools for early detection of hereditary cancer predisposition. These tools could enable clinicians to identify individuals at high risk for developing cancer, allowing for proactive interventions and preventative measures.
Moreover, these diagnostic tools can be used to monitor the effectiveness of gene editing therapies in clinical trials. By tracking the presence and impact of the edited genes, researchers can gain valuable insights into the mechanisms of action and potential side effects of these therapies. This data will be essential for refining gene editing strategies and optimizing treatment protocols for maximum efficacy and safety.
Clinical Trials and Ethical Considerations
The successful translation of gene editing technologies into clinical practice necessitates the conduct of rigorous clinical trials. These trials must carefully evaluate the safety and efficacy of gene editing therapies in patients with hereditary cancers, while addressing potential risks associated with off-target effects and immune responses. Ethical considerations surrounding gene editing, particularly in germline cells, must be carefully considered and addressed by regulatory bodies and research institutions.
Informed consent processes for patients and families must be transparent and comprehensive, ensuring that individuals understand the potential benefits and risks associated with gene editing interventions. Ongoing dialogue between researchers, clinicians, ethicists, and patients is crucial for developing responsible and ethically sound guidelines for the implementation of gene editing therapies in the clinic. A robust regulatory framework is essential to ensure the safe and responsible development and application of gene editing technologies.
Optimizing Delivery Mechanisms
Efficient delivery of gene-editing components to target cells is a critical aspect of gene editing therapies for hereditary cancers. The development of novel delivery systems, such as nanoparticles and viral vectors, is crucial for improving the efficacy and safety of these therapies. Optimizing the delivery of CRISPR-Cas9 complexes to specific cells within the body, while minimizing off-target effects, is a significant hurdle that must be overcome for widespread clinical adoption.
Researchers are actively exploring various delivery methods to enhance the targeting and intracellular delivery of gene editing tools. Factors such as size, charge, and biodegradability of delivery systems need careful consideration to ensure efficient delivery to the desired cells, while minimizing potential toxicity and adverse effects. These advancements in delivery mechanisms are essential for maximizing the therapeutic potential of gene editing and making it a viable treatment option for a wider range of hereditary cancers.
Long-Term Monitoring and Follow-up
Long-term monitoring and follow-up are essential components of clinical trials involving gene editing therapies for hereditary cancers. Careful observation of patients receiving gene editing interventions is crucial for assessing the long-term effects of these therapies, including potential delayed or unexpected side effects. Data collection and analysis from long-term follow-up studies will be essential for understanding the safety profile and efficacy of these therapies in the context of the natural history of the underlying cancers. This information will be critical for informing ongoing research and development efforts, ultimately leading to improved clinical outcomes and reduced morbidity and mortality.
Establishing robust, standardized monitoring protocols is essential for ensuring the collection of high-quality data. These protocols should include regular assessments of physical health, genetic profiles, and tumor markers to detect any adverse effects or recurrence of the cancer. This comprehensive approach to long-term monitoring is critical for evaluating the long-term efficacy and safety of gene editing therapies and their impact on the quality of life for patients with hereditary cancers.