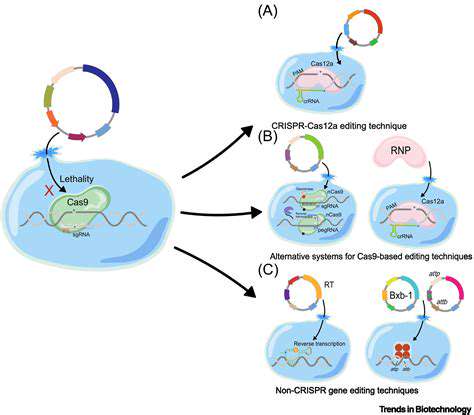
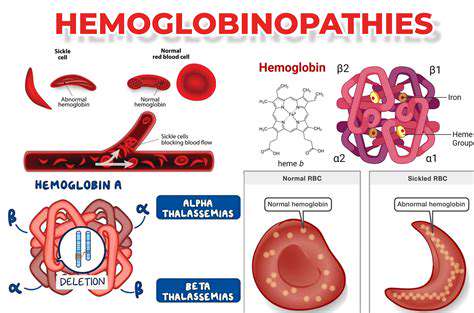
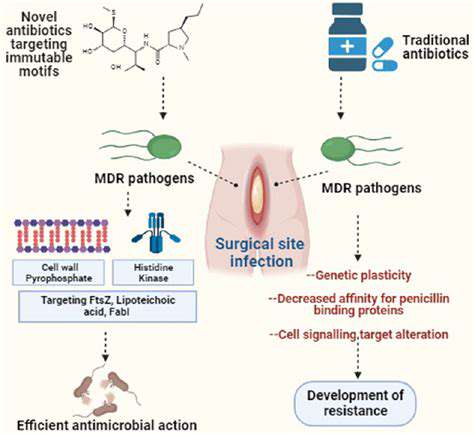
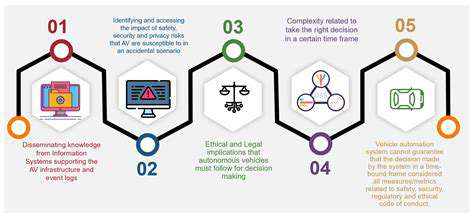
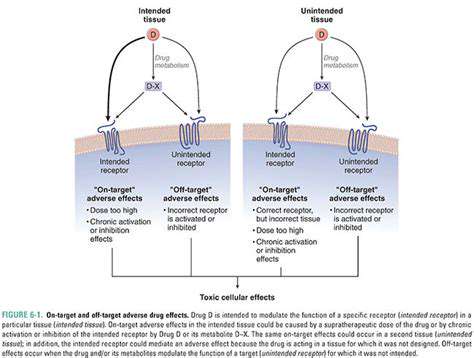
Beyond the Basics: Zinc Finger Nucleases (ZFNs)
Zinc finger nucleases (ZFNs) represent an early attempt at programmable gene editing, predating CRISPR-Cas9. These tools utilize engineered zinc finger proteins that bind to specific DNA sequences, directing the action of a nuclease enzyme to create a double-strand break. While ZFNs demonstrated the feasibility of targeted gene editing, their design and development process were significantly more complex and time-consuming compared to CRISPR-Cas9. This complexity often hindered their widespread adoption, although recent advancements in the field promise to address some of these limitations.
ZFNs offer a degree of versatility in targeting different genomic locations. However, the inherent complexity of designing and testing these proteins for optimal performance remains a challenge. Precise targeting and minimizing off-target effects are crucial for effective and safe gene editing, and ZFNs still require substantial optimization in these areas.
TALENs: Transcription Activator-Like Effector Nucleases
Transcription activator-like effector nucleases (TALENs) emerged as another gene editing tool, built upon the principles of modularity. Similar to ZFNs, TALENs utilize engineered proteins to bind to specific DNA sequences. However, the modular nature of TALENs' DNA-binding domains makes them potentially easier to engineer and tailor for specific targets. This modularity simplifies the design process, reducing the time and effort required to generate custom TALENs compared to ZFNs.
Despite this advantage, the development and optimization of TALENs still required a significant amount of research and testing. Their efficacy and safety profile were also subject to scrutiny, which ultimately contributed to the rise of CRISPR-Cas9 as the preferred gene editing tool in many applications.
Meganucleases: The Pioneers of Gene Editing
Meganucleases, a family of naturally occurring enzymes, are the true pioneers in targeted gene editing. These large proteins recognize and cleave specific DNA sequences, often quite long. The inherent specificity of meganucleases makes them valuable tools for gene editing, especially in situations where precise targeting is paramount. While their recognition sequence lengths can present a challenge in terms of design and targeting efficiency, the inherent specificity of meganucleases provides a foundation for potentially more efficient and safer gene editing.
Base Editing and Prime Editing: Beyond Double-Strand Breaks
Base editing and prime editing represent a significant evolution in gene editing technology. Instead of creating double-strand breaks, these methods directly modify individual DNA bases, thereby offering a potentially more precise and less disruptive approach. This precise, base-level editing capability holds enormous potential for treating genetic diseases and studying gene function without the inherent risks associated with double-strand break-induced DNA repair pathways. Scientists are actively exploring the potential of these techniques for more efficient and safer gene therapy.
Base editing and prime editing introduce a new paradigm in gene editing, offering the prospect of more precise and targeted modifications. The potential to directly modify individual bases holds the promise of a less invasive and potentially more effective approach to treating a wider range of genetic disorders. However, ongoing research is vital to fully understand the potential benefits and risks associated with these innovative techniques.
RNA-Guided Nucleases: A Diverse Family Beyond CRISPR-Cas9
Beyond CRISPR-Cas9, other RNA-guided nucleases are emerging as potential gene editing tools. These nucleases utilize RNA molecules to recognize specific DNA sequences, similar to CRISPR-Cas9's mechanism. Different RNA-guided nucleases offer unique characteristics in terms of specificity, efficiency, and potential applications. This diversity offers a range of options for researchers to explore and potentially adapt for specific gene editing needs.
Gene Editing in the Future: Promising Directions
The future of gene editing holds immense promise, with ongoing research exploring new methods and expanding the scope of applications. Researchers are continuously developing and refining existing tools, while also discovering entirely new approaches. The long-term impact of gene editing technologies on human health and our understanding of biology is likely to be profound. The potential to treat genetic diseases, enhance crop yields, and even engineer organisms for specific purposes promises a future revolutionized by the power of gene editing.
Mixed reality (MR) environments offer a unique opportunity to bridge the gap between theoretical knowledge and practical application. By overlaying digital information onto the real world, MR allows learners to interact with concepts in a way that traditional methods simply cannot replicate. This immersive experience fosters a deeper understanding and retention of complex information, as learners can visualize and manipulate objects, processes, and systems in a dynamic, engaging context.