Defining the Drug Discovery Pipeline Landscape
Understanding the Stages of a Drug Discovery Pipeline
A drug discovery pipeline is a multi-stage process that takes promising molecules from initial research and development to eventual clinical trials and, hopefully, the market. This intricate journey involves a series of well-defined steps, each requiring rigorous testing and evaluation to ensure safety and efficacy. Understanding these stages, from target identification to preclinical testing and clinical trials, is crucial for optimizing the process and maximizing the chances of bringing a successful drug to market. This comprehensive approach, while often lengthy and complex, is essential for ensuring patient safety and the development of effective medications.
Each stage of a drug discovery pipeline presents unique challenges and opportunities. Careful consideration of potential obstacles and innovative strategies to overcome them is paramount for efficiently navigating the process. This includes the selection of appropriate research methodologies, the utilization of advanced technologies, and the recruitment of skilled professionals with expertise in various disciplines.
Target Identification and Validation
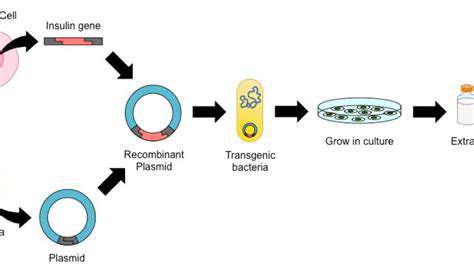
Identifying and validating potential drug targets is a critical first step in the drug discovery process. This involves meticulous research into biological pathways and disease mechanisms to pinpoint molecules or cellular components that are crucial for the progression of the disease. Extensive research, encompassing literature reviews, laboratory experiments, and computational modeling, is employed to identify and validate these targets. The selection of a suitable target is crucial, as it dictates the direction of the entire drug discovery pipeline and influences the success rate of the project.
Preclinical Testing and Development
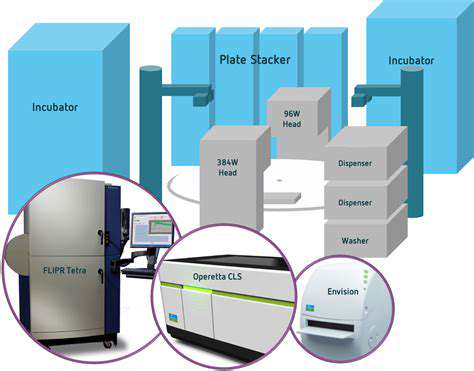
Once a target is identified, preclinical studies are conducted to evaluate the safety and efficacy of potential drug candidates. This stage involves extensive laboratory and animal testing to assess the drug's pharmacokinetic properties, potential side effects, and overall therapeutic effect. The results of these studies are used to support the decision-making process and guide the design of clinical trials. Preclinical testing is critical for ensuring the safety of a drug candidate before it's administered to humans.
This phase often involves in vitro and in vivo studies, using various animal models to mimic human physiology. Rigorous analysis of the data collected in these experiments is essential for determining the potential of a drug candidate and informing the next steps in the pipeline.
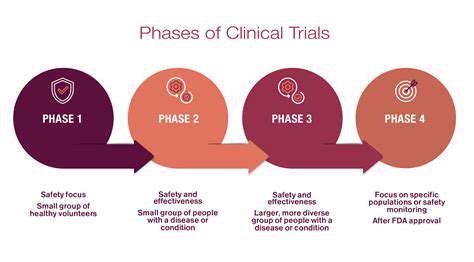
Clinical Trials and Regulatory Approval
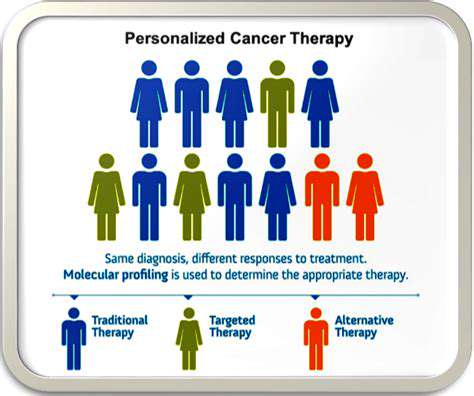
Clinical trials are the crucial stage where potential drugs are tested on human subjects to assess their safety and efficacy in a controlled environment. These trials are typically conducted in phases, progressing from small-scale studies to large-scale trials involving thousands of patients. The results of these trials, meticulously analyzed and documented, are submitted to regulatory agencies for approval. The process of obtaining regulatory approval is rigorous and demands extensive evidence of a drug's safety and effectiveness. This rigorous process is essential to protect public health and ensure that only safe and effective drugs reach the market.
Regulatory approval is a significant milestone in the drug discovery pipeline, marking the transition from research to a potential new treatment option for patients. This step often involves extensive interactions with regulatory bodies, requiring careful adherence to guidelines and regulations throughout the entire process.