The Future of Biotechnological Interventions for Gut Health
Personalized Gut Microbiome Interventions
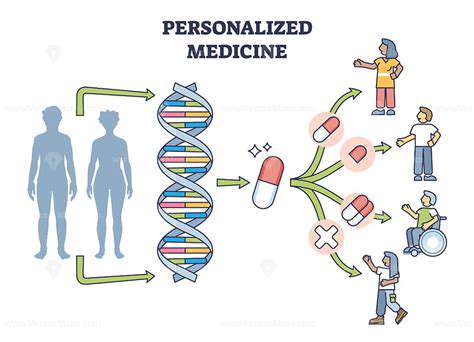
The future of biotechnological interventions for gut health hinges significantly on the ability to personalize treatments. Current research is focused on understanding the intricate interplay between individual genetic predispositions, dietary habits, and the unique composition of an individual's gut microbiome. This personalized approach will allow for the development of targeted interventions, potentially circumventing the limitations of 'one-size-fits-all' strategies. Scientists are exploring the use of advanced sequencing technologies to analyze individual gut microbiomes, identifying specific bacterial species or metabolites that might be associated with specific health conditions. This tailored approach promises to maximize the effectiveness of biotechnological solutions, potentially leading to more effective and safer treatments.
Furthermore, personalized interventions could involve the development of customized prebiotics and probiotics. These targeted formulations could be designed to address specific imbalances in the gut microbiome, bolstering beneficial bacteria and mitigating harmful ones. This customized approach could revolutionize the treatment of digestive disorders, allergies, and potentially even mental health conditions that have been linked to gut dysbiosis. The ability to fine-tune interventions based on an individual's unique profile will be crucial for optimizing the effectiveness of these interventions.
Gene Editing and Microbial Engineering
Advancements in gene editing technologies, like CRISPR-Cas9, offer a powerful tool for manipulating the gut microbiome. These technologies allow scientists to potentially modify specific bacteria, either to enhance beneficial functions or to eliminate harmful ones. This could lead to interventions that directly target the root causes of specific gut-related diseases, rather than just managing symptoms. The potential to directly modulate the expression of genes within specific bacterial species holds great promise for manipulating their metabolic functions and interactions within the gut ecosystem.
Beyond gene editing, microbial engineering holds the potential to create novel bacterial strains with enhanced therapeutic properties. These engineered microbes could be designed to produce specific compounds that promote gut health, such as short-chain fatty acids (SCFAs). This approach could lead to the development of novel therapies for inflammatory bowel diseases, obesity, and other conditions linked to an unhealthy gut microbiome. This opens up avenues to develop truly transformative treatments that go beyond simple supplementation.
Novel Delivery Systems and Targeted Therapies
Developing innovative delivery systems for biotechnological interventions is crucial to their effectiveness. Researchers are exploring novel methods to deliver probiotics, prebiotics, or even gene-edited bacteria directly to the gut. This targeted delivery aims to improve the survival and colonization of beneficial microbes within the digestive tract, minimizing the need for multiple daily doses and potentially improving patient compliance. Nanotechnology and microencapsulation are prominent avenues that researchers are exploring for targeted delivery, allowing for controlled release and enhanced bioavailability of therapeutic agents.
Furthermore, the development of targeted therapies that specifically address the mechanisms underlying gut-related diseases will be critical. These interventions could focus on restoring the balance of the gut microbiome, modulating inflammation, or improving nutrient absorption. The ongoing pursuit of understanding complex interactions within the gut ecosystem will lead to the development of more effective and less invasive treatments. This targeted approach could dramatically improve outcomes for patients suffering from a wide range of gut-related disorders.
Diagnostics and Monitoring
Effective biotechnological interventions for gut health necessitate accurate and reliable diagnostics. The development of rapid, cost-effective, and non-invasive methods to assess the gut microbiome composition and function is essential. This includes the development of advanced diagnostic tools that can identify specific biomarkers associated with different gut conditions, enabling early detection and personalized treatment strategies. This will lead to earlier intervention and potentially prevent the progression of debilitating diseases.