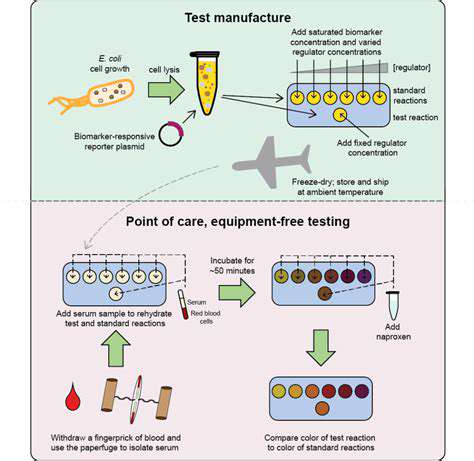
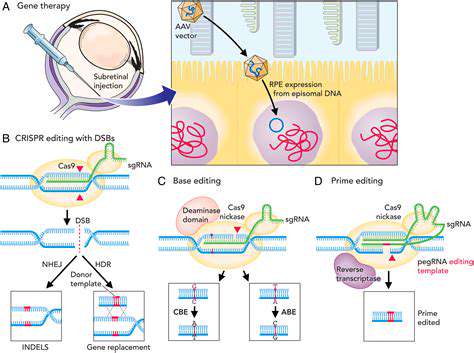
Introduction to Synthetic Biology in Drug Delivery

Defining Synthetic Biology
Synthetic biology is a rapidly evolving field that combines engineering principles with biological systems. It aims to design and construct novel biological parts, devices, and systems, and to redesign existing biological systems for useful purposes. This interdisciplinary approach leverages our understanding of biology to create solutions in various sectors, from medicine to energy production.
At its core, Synthetic biology involves manipulating genetic material and cellular processes to achieve specific outcomes. This often involves creating new genetic circuits, modifying existing ones, or even assembling entirely new biological pathways.
Key Concepts in Synthetic Biology
Fundamental to synthetic biology are the concepts of modularity and standardization. Modularity allows scientists to design and assemble biological systems from interchangeable parts, much like building with LEGO bricks. Standardization ensures that these parts can be readily shared and used across different projects, accelerating research and development. This modularity and standardization are essential for the efficient design and construction of complex biological systems.
Applications of Synthetic Biology
The applications of synthetic biology are vast and span across numerous fields. In medicine, for example, it holds promise for developing novel therapies, creating personalized medicine approaches, and enhancing our understanding of disease mechanisms. Synthetic biology is also revolutionizing the field of energy production by enabling the development of biofuels and more efficient bio-based materials.
Furthermore, advancements in synthetic biology are leading to innovative solutions in agriculture, environmental remediation, and industrial biotechnology.
Tools and Technologies in Synthetic Biology
Several powerful tools and technologies underpin synthetic biology research. These include DNA synthesis, gene editing tools like CRISPR-Cas9, and high-throughput screening methods. These technologies allow scientists to rapidly design, construct, and test biological systems, accelerating the pace of innovation.
Ethical Considerations in Synthetic Biology
As synthetic biology advances, it's crucial to consider the ethical implications of this powerful technology. Concerns about unintended consequences, the potential for misuse, and the equitable distribution of benefits need careful consideration. Open and transparent discussions are necessary to ensure that synthetic biology is developed and applied responsibly, benefiting society as a whole.
These considerations extend to the potential impacts on biodiversity and the environment, necessitating thoughtful strategies for risk assessment and management.
The Future of Synthetic Biology
The future of synthetic biology is bright, promising breakthroughs in various fields. Further research and development are expected to lead to more sophisticated biological systems with enhanced capabilities and functionalities. This will undoubtedly revolutionize our understanding of life and pave the way for novel solutions to complex global challenges.
Expect further integration with other fields like nanotechnology and artificial intelligence, leading to even more groundbreaking applications in the years to come.
Engineering Microbial Factories for Drug Delivery
Harnessing Microbial Potential
Synthetic biology, a rapidly evolving field, offers a unique opportunity to engineer microorganisms into sophisticated drug delivery systems. By reprogramming the genetic blueprints of bacteria, yeast, or other suitable organisms, scientists can tailor their metabolic pathways to produce and secrete therapeutic compounds. This approach bypasses traditional chemical synthesis methods, potentially offering significant cost savings and environmental benefits. Moreover, it allows for the development of highly specific and targeted drug delivery systems, enhancing efficacy and reducing side effects.
Harnessing the natural capabilities of microorganisms for drug production is not a new concept, but synthetic biology empowers researchers to fine-tune and optimize these processes. This includes increasing the yield of desired products, improving their stability, and even modifying their release kinetics to achieve optimal therapeutic outcomes.
Designing Microbial Drug Carriers
One crucial aspect of engineering microbial factories for drug delivery is designing the microorganisms to act as sophisticated drug carriers. This involves modifying the microbial cell surface to enhance its interactions with the target cells in the body. Furthermore, scientists can engineer the microbial cells to release the drugs at specific locations or in response to specific stimuli, ensuring optimal therapeutic efficacy.
These engineered microbial carriers can be designed to target specific tissues or organs, thus minimizing off-target effects. The precise control over the release mechanism is paramount for effective drug delivery, allowing for sustained drug release and potentially reducing the frequency of administrations.
Optimizing Metabolic Pathways
A key element in creating effective microbial drug factories lies in optimizing the metabolic pathways responsible for drug production. This often involves identifying and modifying specific enzymes within the microbial cell to enhance the production rate and efficiency of the desired drug molecule. Precise genetic manipulation allows researchers to modify the cell's internal machinery to maximize the synthesis of the therapeutic agent.
Optimizing metabolic flux through metabolic engineering techniques can lead to increased production yields. This requires a detailed understanding of the microbial metabolism and the identification of bottlenecks in the pathway, allowing targeted interventions to increase production.
Controlling Drug Release Kinetics
The ability to control the release kinetics of drugs from the microbial factory is critical for therapeutic efficacy. Scientists can manipulate the genetic components within the microorganism to control the rate at which the drug is released. This enables the design of systems that release drugs in a sustained manner, mimicking the natural release patterns of hormones or other bioactive molecules.
Precise control over drug release is crucial to avoid sudden surges or depletions in drug concentration. This ensures a consistent and predictable therapeutic response, minimizing potential side effects and maximizing efficacy.
Addressing Safety and Biocompatibility Concerns
Ensuring the safety and biocompatibility of engineered microbial drug delivery systems is paramount. Rigorous testing and characterization of the engineered microorganisms are essential to minimize any potential risks associated with their introduction into the human body. This includes evaluating the potential for toxicity, immunogenicity, and the possibility of unintended interactions with the host's immune system.
Thorough safety assessments are crucial to prevent adverse reactions and ensure the long-term viability and efficacy of the drug delivery system. This involves assessing the potential for the engineered microorganism to interact with other components within the body and evaluating the potential for the development of resistance mechanisms.
Scaling Up Production and Manufacturing
Successfully translating laboratory-based research into a viable and scalable manufacturing process is a significant challenge in synthetic biology. Developing efficient and cost-effective methods for large-scale cultivation and harvesting of the engineered microorganisms is necessary for widespread clinical application. This includes optimization of the fermentation processes and the development of robust purification techniques.
Scaling up production requires careful consideration of factors like nutrient requirements, oxygen supply, and temperature control during fermentation. The development of automated systems for large-scale production is crucial to ensure consistency and quality control, making the process economically viable.
Creating Bioresponsive Drug Carriers

Harnessing the Power of Bioresponsive Materials
Bioresponsive drug carriers are designed to release their therapeutic payload in a controlled and targeted manner, responding to specific stimuli within the body. This precise release mechanism is crucial for maximizing drug efficacy and minimizing side effects. By responding to physiological changes, such as pH or temperature fluctuations, these carriers can release the drug only when and where it's needed, avoiding unnecessary exposure to healthy tissues. This strategy enhances the therapeutic index of the drug.
These materials often mimic natural biological processes, allowing for a more efficient and natural delivery system. The careful selection of bioresponsive components is essential for achieving the desired release profile and ensuring compatibility with the biological environment.
Types of Bioresponsive Triggers
A variety of triggers can be used to activate the release of the drug. These triggers often include pH changes, temperature fluctuations, or the presence of specific enzymes or proteins. Understanding the specific physiological conditions in the target tissue is vital for selecting the appropriate trigger. This ensures that the drug is released only when and where it's needed, maximizing its therapeutic effect and minimizing its side effects.
For example, pH-sensitive polymers can release drugs in response to the acidic environment of tumors, while temperature-sensitive polymers may release drugs in response to the elevated temperature of inflamed tissues.
Advantages of Bioresponsive Drug Delivery
Bioresponsive drug delivery systems offer several key advantages over traditional drug delivery methods. These advantages include improved drug efficacy, reduced side effects, and enhanced targeting of the therapeutic agent to the diseased tissue.
Precise control over drug release is achievable, leading to optimal therapeutic outcomes and minimizing the risk of adverse reactions. This tailored approach to medication administration is a significant advancement in pharmaceutical science.
Challenges in Development
Despite the significant advantages, challenges remain in the development of bioresponsive drug carriers. These challenges include the need for precise control over the release mechanism and the ensuring compatibility with the biological environment. Careful consideration of the biocompatibility and potential immunogenicity of the materials is crucial for successful clinical translation.
Ensuring the drug's stability and maintaining its activity throughout the delivery process are also significant concerns that require careful attention. Optimizing the design of these carriers for specific therapeutic applications is essential for widespread clinical adoption.
Drug Loading and Release Mechanisms
The effective loading of drugs into bioresponsive carriers is crucial for their efficacy. Various strategies are employed to achieve this, such as encapsulation, adsorption, and covalent conjugation. The chosen method should ensure the drug's stability and maintain its therapeutic activity throughout the delivery process. Careful optimization of these loading methods is vital for successful drug delivery.
The release mechanism is equally critical. The responsive nature of the carrier material should be precisely controlled to ensure the targeted and timely release of the drug. This allows for the drug to be delivered at the optimal concentration and time, maximizing its efficacy and minimizing side effects.
Clinical Applications and Future Directions
Bioresponsive drug carriers hold immense promise for various clinical applications, including cancer therapy, targeted gene delivery, and treating inflammatory diseases. The ability to precisely control the drug release in response to specific stimuli in the body opens up exciting possibilities for personalized medicine.
Future research will focus on enhancing the biocompatibility, targeting specificity, and controlled release profiles of these carriers. Further investigations into the complex interactions between bioresponsive materials and biological systems are crucial to maximize the therapeutic potential of these innovative drug delivery systems.
Regulatory Considerations
The development and implementation of bioresponsive drug carriers require careful consideration of regulatory guidelines. Thorough preclinical and clinical studies are essential to demonstrate the safety and efficacy of these novel drug delivery systems. Strict adherence to regulatory requirements is essential for ensuring patient safety and the ethical conduct of research and development.
Meeting these standards is essential to obtain regulatory approvals and ensure the safe and effective use of these advanced therapies. This ensures that these promising technologies are implemented responsibly and benefit patients.
Developing Cellular Delivery Systems
Harnessing Synthetic Biology for Targeted Drug Delivery
Synthetic biology offers a revolutionary approach to designing and engineering cellular delivery systems. By leveraging the power of genetic engineering, researchers can tailor cellular components to precisely control drug release and delivery, leading to increased efficacy and reduced side effects. This innovative approach allows for the creation of smart drug delivery systems, capable of responding to specific stimuli and releasing drugs at the desired location and time.
This targeted approach is crucial for improving the treatment of various diseases. By delivering drugs directly to the affected cells, synthetic biology-based systems minimize the exposure of healthy tissues to potentially harmful drugs, thus reducing side effects and improving patient outcomes.
Engineering Cellular Vehicles for Drug Transport
A key aspect of developing cellular delivery systems involves engineering specialized cellular vehicles to effectively transport drugs to their intended targets. This could involve modifying the cell's membrane permeability, creating intracellular compartments for drug storage, or even developing entirely new cellular structures designed for drug encapsulation and release.
The design of these cellular vehicles needs to consider factors such as the specific drug being delivered, the target tissue, and the desired release mechanism. The design process often involves extensive computational modeling and experimental validation to ensure the vehicle's functionality and safety.
Controlling Drug Release with Cellular Signaling
Advanced cellular delivery systems can be programmed to release drugs in response to specific cellular signals. This allows for a more precise and controlled drug release, maximizing therapeutic efficacy and minimizing potential harm to healthy tissues.
For instance, the system might be designed to release the drug only when a specific enzyme is present, or when the cell's environment reaches a certain pH level. Such systems offer a significant advancement in personalized medicine, tailoring treatment to individual patient needs.
Designing Responsive Cellular Systems
Developing cellular delivery systems that respond to external stimuli, such as light or temperature changes, allows for remote control over drug release. This opens up exciting possibilities for treating diseases in a more targeted and controlled manner.
Imagine a system that releases a drug only when a specific light wavelength is applied, or a system that triggers drug release at a particular temperature. Such responsive systems can be extremely valuable in scenarios where precise timing and location of drug delivery are paramount.
Optimizing Cellular Uptake and Trafficking
Efficient cellular uptake and trafficking of the drug-carrying cells are critical for successful delivery. Researchers need to consider factors such as the cell type, the drug's chemical properties, and the physiological environment to optimize cellular uptake and intracellular trafficking.
Strategies to enhance cellular uptake could include modifying the drug's surface properties, attaching targeting ligands to the cell, or using biocompatible materials to facilitate cellular recognition and internalization.
Addressing Challenges in Cellular Delivery
Despite the potential benefits of cellular delivery systems, several challenges remain. Ensuring the safety and biocompatibility of the engineered cells is paramount. Furthermore, factors like the immune response to the modified cells and the potential for off-target effects need careful consideration.
Addressing these challenges requires rigorous testing and optimization of the delivery systems. Safety and efficacy are paramount in ensuring the responsible application of these innovative technologies in clinical settings.
Future Directions and Applications
The future of cellular delivery systems holds immense promise in various therapeutic applications, including cancer treatment, gene therapy, and regenerative medicine. Further research and development are essential to overcome existing challenges and translate these technologies into effective clinical tools.
Exploring novel cellular types, developing more sophisticated control mechanisms, and optimizing drug release kinetics are crucial steps in advancing this field. Ultimately, these advancements could lead to more personalized and effective treatments for a wide range of diseases.
Future Directions and Challenges
Emerging Technologies and Innovation
The rapid advancement of artificial intelligence (AI) and machine learning (ML) presents exciting opportunities for the future of our field. These technologies can automate complex tasks, potentially leading to significant efficiency gains and breakthroughs in various applications. AI-powered tools can analyze vast datasets to identify patterns and insights that would be impossible for humans to discern, revolutionizing how we approach problem-solving.
Furthermore, advancements in quantum computing promise to unlock entirely new levels of computational power, enabling us to tackle problems currently considered intractable. This could lead to breakthroughs in materials science, drug discovery, and fundamental scientific research.
Ethical Considerations and Responsible Use
As AI and related technologies become more integrated into our lives, it is crucial to address the ethical implications. Ensuring fairness, transparency, and accountability in the development and deployment of these technologies is paramount. We must carefully consider the potential biases that can be embedded in algorithms and take steps to mitigate their impact.
Furthermore, questions of privacy and data security need to be proactively addressed. Protecting sensitive information and ensuring responsible data handling practices are vital to building trust and ensuring the safe and beneficial use of these powerful tools.
Data Management and Accessibility
The sheer volume of data generated daily requires robust data management systems and infrastructure. Efficient storage, processing, and retrieval mechanisms are critical for extracting meaningful insights and knowledge from this vast ocean of information. Developing standardized protocols and interoperable data formats will be key to achieving widespread access and collaboration among researchers.
Furthermore, ensuring the accessibility of data for diverse stakeholders, including researchers, policymakers, and the public, is essential for promoting transparency and fostering informed decision-making. This will allow for wider adoption and broader impact of the advancements made.
Collaboration and Knowledge Sharing
Effective collaboration between researchers, industry partners, and policymakers is essential for navigating the complexities of the future. Open communication and shared knowledge will accelerate progress and ensure that innovations are deployed responsibly and effectively. Cross-disciplinary collaborations will be critical for addressing complex problems and leveraging the diverse perspectives of experts from various fields.
Furthermore, fostering a culture of knowledge sharing through publications, conferences, and online platforms is crucial for disseminating important findings and fostering a global community of researchers.
Resource Allocation and Funding
Adequate resources, including funding for research, development, and infrastructure, are crucial for realizing the full potential of future directions in our field. Strategic investment in emerging technologies and human capital will be essential to maintain a competitive edge. Prioritizing research areas with high societal impact and addressing the needs of underserved populations will ensure that advancements benefit everyone.
Maintaining a Focus on Human Well-being
Ultimately, any advancements in technology must be evaluated through the lens of human well-being. Considerations of social impact, equity, and inclusivity are essential components of responsible innovation. We must strive to ensure that technological progress leads to a more equitable and prosperous future for all.
Furthermore, understanding the potential societal consequences of new technologies and proactively addressing potential challenges are vital for ensuring that these advancements serve humanity's best interests.