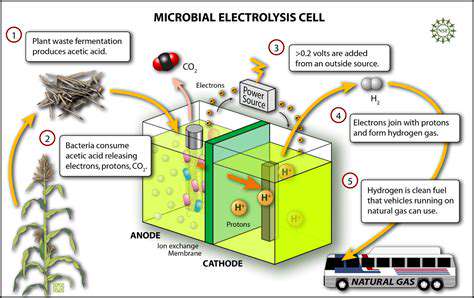
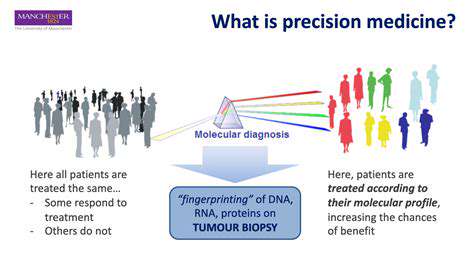
Introduction to Synthetic Biology in Diagnostics

Defining Synthetic Biology
Synthetic biology is an interdisciplinary field of science that aims to design and construct new biological parts, devices, and systems, or to redesign existing natural biological systems. It leverages principles from engineering, computer science, and other disciplines to create novel functionalities in living organisms. This field is rapidly evolving, offering innovative solutions to global challenges. This approach focuses on understanding and re-engineering biological processes for practical applications.
It involves assembling genetic components into novel combinations to create organisms with enhanced or novel capabilities. This process, similar to building with LEGOs on a cellular level, allows for the creation of organisms with custom-designed functions. The potential applications are vast, ranging from biofuels production to disease treatment.
Key Concepts in Synthetic Biology
Central to synthetic biology are the concepts of modularity and standardization. Modularity allows for the assembly of biological components in a predictable and controllable manner, much like constructing a mechanical device from pre-made parts. This approach simplifies design and optimization, akin to designing a car using standardized components.
Standardization, in turn, ensures that these components are compatible and interchangeable, facilitating the construction of more complex systems. This is crucial for reproducibility and scalability in synthetic biology experiments.
Applications of Synthetic Biology
The applications of synthetic biology are wide-ranging, impacting various sectors. One key application is the development of biofuels from renewable resources. This can significantly reduce our dependence on fossil fuels and contribute to a more sustainable energy future. This innovative approach to fuel production is a promising step in environmental sustainability.
Another application lies in the creation of bio-based materials and products. Synthetic biology holds the potential to produce bioplastics, bio-based textiles, and other materials with reduced environmental impacts compared to traditional petroleum-derived products. This is a significant area of research with substantial potential for industrial applications.
Tools and Technologies in Synthetic Biology
Several tools and technologies are essential for synthetic biology research. These include genetic engineering techniques such as CRISPR-Cas9, which allow precise editing of DNA sequences. This technology enables scientists to modify organisms with targeted changes, leading to more controlled outcomes.
Advanced computational tools are also crucial for designing and optimizing biological systems. These tools simulate and predict the behavior of biological systems, accelerating the design process and reducing the need for extensive experimentation.
Ethical Considerations in Synthetic Biology
As with any rapidly advancing technology, synthetic biology raises important ethical considerations. Concerns regarding the potential misuse of this technology must be addressed. Careful consideration of potential risks and benefits is essential for responsible development and application.
Public engagement and transparent communication are critical to fostering trust and addressing public anxieties. This includes open discussions about potential risks and benefits, ensuring that the technology is developed and applied ethically and responsibly.
The Future of Synthetic Biology
The future of synthetic biology promises exciting advancements across numerous fields. With continued research and development, synthetic biology could lead to breakthroughs in medicine, agriculture, and environmental remediation. The innovative potential of this field is immense, and its impact on our lives is expected to be transformative.
This field is rapidly evolving, leading to new discoveries and applications that will continue to shape our world in the years ahead. The potential for progress in diverse areas is vast.
Current Research Trends
Current research in synthetic biology is focused on improving the efficiency and scalability of biological systems. Researchers are exploring ways to optimize metabolic pathways to produce desired products more effectively.
Another important trend is the development of more sophisticated tools for genetic engineering and biological design. This includes advancements in DNA synthesis, assembly, and characterization technologies. This is crucial for further advancements in the field.
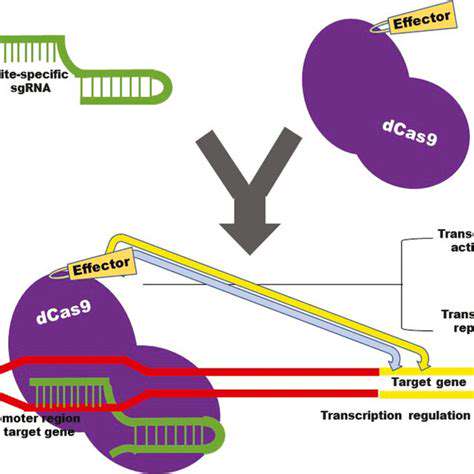
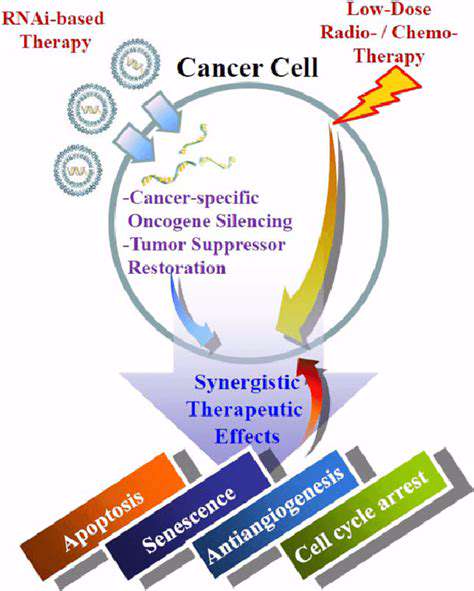
Developing CRISPR-Cas Systems for Diagnostics
Harnessing CRISPR-Cas for Rapid Detection
CRISPR-Cas systems, derived from bacterial adaptive immune systems, are revolutionizing diagnostic tools due to their remarkable potential for rapid and sensitive detection of pathogens and genetic mutations. These systems offer a powerful alternative to traditional methods, promising faster turnaround times, reduced costs, and enhanced portability. The ability to design specific CRISPR-Cas systems targeting unique nucleic acid sequences allows for highly selective detection, minimizing false positives and ensuring accurate results. This specificity is crucial in clinical settings where precise identification of pathogens or disease markers is paramount for effective treatment and patient management.
The development of CRISPR-Cas-based diagnostic platforms is progressing rapidly, with various innovative approaches being explored. Researchers are optimizing the CRISPR-Cas components, such as the guide RNA and Cas enzyme, to enhance sensitivity and specificity. This includes engineering Cas enzymes with improved activity and minimizing off-target effects. Furthermore, the miniaturization of these systems into portable devices is a key area of focus, opening avenues for point-of-care diagnostics in remote or resource-limited settings.
Expanding Applications in Point-of-Care Diagnostics
The potential applications of CRISPR-Cas systems in point-of-care diagnostics are vast and rapidly expanding. Beyond pathogen detection, these systems can be adapted for the detection of genetic mutations associated with various diseases, including cancers. Early detection of these mutations can lead to earlier interventions, potentially improving patient outcomes. Imagine a future where clinicians can rapidly identify infectious agents or genetic abnormalities directly at the patient's bedside, enabling prompt and targeted treatment decisions. This translates into significant advantages in terms of speed, efficiency, and accessibility of diagnostic information.
The development of CRISPR-Cas-based diagnostic platforms is particularly promising for infectious disease surveillance, allowing for rapid identification of emerging pathogens. This real-time monitoring can be vital in controlling outbreaks and guiding public health interventions. Moreover, these systems can be adapted for the detection of environmental contaminants, offering a powerful tool for environmental monitoring and safeguarding public health.
Optimizing CRISPR-Cas for Multiplexing and Automation
A key challenge in expanding the utility of CRISPR-Cas diagnostics is the need for multiplexing capabilities. The ability to detect multiple targets simultaneously in a single reaction would greatly enhance the efficiency and information content of diagnostic assays. Research is focused on developing strategies for multiplexing CRISPR-Cas systems, enabling parallel detection of various pathogens or genetic markers. This approach promises a significant boost in diagnostic throughput, allowing for more comprehensive assessments in a shorter period.
The integration of CRISPR-Cas systems with automated platforms is another crucial aspect of their development for widespread clinical application. Automated systems can streamline sample processing, reduce manual errors, and increase overall efficiency, paving the way for high-throughput diagnostics. These automation strategies will be critical for widespread adoption in clinical laboratories and point-of-care settings.
Integrating microfluidic devices with CRISPR-Cas technology will further enhance the capabilities of these diagnostic platforms. Microfluidic channels can precisely control sample flow, enabling miniaturization and cost-effectiveness. This integration holds immense potential for creating highly sensitive and portable diagnostic devices that can operate with minimal sample volumes, further expanding the reach and accessibility of CRISPR-Cas-based diagnostics.