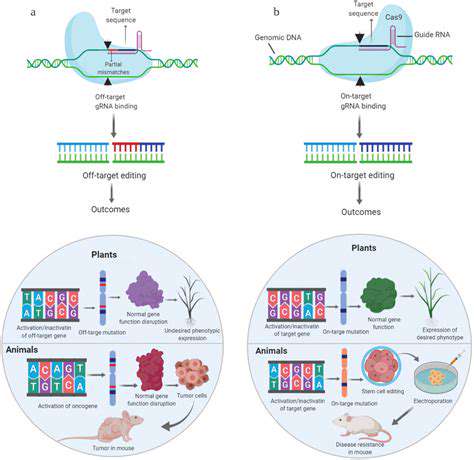
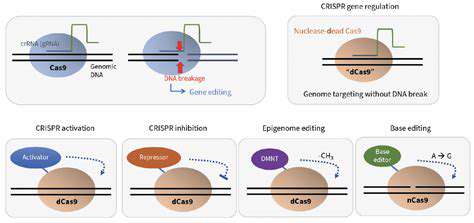
While CRISPR-Cas9 has undoubtedly revolutionized gene editing, ongoing research is actively exploring and developing even more sophisticated techniques. These advancements are pushing the boundaries of what's possible, offering greater precision, efficiency, and safety. Researchers are investigating alternative gene-editing tools, such as base editors and prime editors, which can modify DNA without causing double-strand breaks. This approach holds significant promise for reducing potential off-target effects and improving the overall therapeutic profile of gene editing.
The exploration of emerging technologies beyond CRISPR-Cas9 is crucial for advancing the field of gene editing. These new approaches are paving the way for more precise and efficient gene modifications, potentially minimizing the risks associated with gene editing and expanding the spectrum of treatable genetic disorders. Further research and development are essential to fully realize the therapeutic potential of these innovative tools.
These emerging technologies offer the potential to address limitations of CRISPR-Cas9, such as the need for double-strand breaks and potential off-target effects. As research progresses, these new methods could lead to more effective and safer gene therapies, offering hope for a future where genetic diseases are less prevalent and potentially even preventable.
Ethical Considerations and Future Directions
The remarkable potential of gene editing technologies, such as CRISPR-Cas9, necessitates careful consideration of the ethical implications. As these tools become more refined and accessible, it's crucial to establish robust ethical guidelines and regulatory frameworks to ensure responsible application. Open discussions and collaborations between scientists, ethicists, policymakers, and the public are essential to navigate the complex ethical considerations associated with altering the human genome.
The future of gene editing lies in responsible innovation, ensuring that these powerful technologies are used ethically and effectively. Careful monitoring of potential side effects, thorough preclinical testing, and robust clinical trials are crucial to maximize the benefits and minimize the risks of gene therapy. Ongoing dialogue about the societal impact of gene editing is essential to shape its development and deployment in a responsible manner.
Gene Editing Strategies for Disease Prevention
Harnessing CRISPR-Cas9 for Precision Gene Editing
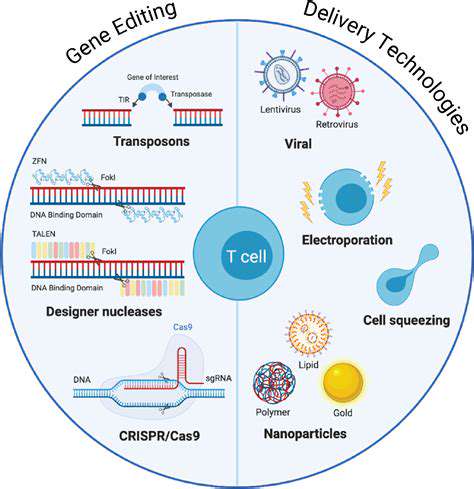
CRISPR-Cas9, a revolutionary gene-editing tool, offers unparalleled precision in targeting and modifying specific DNA sequences. This system, inspired by bacterial defense mechanisms, allows scientists to precisely cut DNA at a desired location, enabling the correction of disease-causing mutations. The potential applications of CRISPR-Cas9 in disease prevention are vast, ranging from correcting single nucleotide polymorphisms (SNPs) linked to inherited diseases to removing entire genes associated with cancer predisposition. Further research is crucial to fully realize the therapeutic potential of CRISPR-Cas9 while addressing safety concerns and optimizing delivery methods.
The ability to precisely target and modify specific genes within the genome holds enormous promise for disease prevention. Researchers are continuously refining CRISPR-Cas9 technology to enhance its efficiency, specificity, and safety profile, paving the way for more effective and targeted therapies. This innovative approach represents a significant advance in our understanding and treatment of genetic diseases.
ZFNs and TALENs: Early Pioneers in Gene Editing
Zinc Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs) were early gene editing tools that preceded CRISPR-Cas9. While they demonstrated the feasibility of targeted gene editing, their application was often more complex and less precise than CRISPR-Cas9. Despite their historical significance, ZFNs and TALENs are now less frequently employed due to the superior characteristics of CRISPR-Cas9.
These early gene editing tools paved the way for the development of CRISPR-Cas9 and contributed significantly to the field's advancement. However, their limitations in terms of design and delivery have led to the preference for CRISPR-Cas9 in contemporary gene editing applications.
Base Editing: Modifying Individual DNA Bases
Base editing is a more recent gene editing approach that focuses on modifying individual DNA bases without creating a double-strand break. This method offers a more precise and potentially safer way to correct mutations, especially for diseases caused by single-base changes. Base editing technology is still under development but has shown promising results in preclinical models and holds significant potential for disease prevention.
Gene Silencing Strategies for Disease Prevention
Gene silencing techniques, such as RNA interference (RNAi), aim to reduce the expression of specific genes linked to disease development. By interfering with the gene's messenger RNA (mRNA) or preventing its translation, RNAi can effectively suppress the production of harmful proteins. This approach could be used to prevent the manifestation of genetic diseases by lowering the levels of faulty proteins.
Gene Therapy Vectors and Delivery Systems
Effective gene editing requires efficient delivery of the gene-editing tools into target cells. Different vectors, such as viral vectors and non-viral nanoparticles, are being developed and tested to improve the delivery of gene-editing components. Finding the optimal delivery systems for various tissues and cell types is crucial for the success of gene editing therapies for disease prevention.
Ethical Considerations in Gene Editing
The potential of gene editing to prevent genetic diseases raises important ethical considerations, including germline editing (editing the genes that are passed on to future generations). Germline editing is a highly controversial topic due to the potential for unintended consequences and the ethical implications of altering the human gene pool. Careful consideration of these ethical concerns is essential as gene editing technologies continue to advance.
Personalized Medicine and Gene Editing
The future of gene editing holds the promise of personalized medicine, tailoring treatments to individual genetic profiles. By analyzing an individual's genome, doctors could identify specific genetic variations linked to disease risk and potentially apply gene editing techniques to address these risks. However, the path towards widespread adoption of this approach will involve overcoming technological and ethical challenges.
Culinary traditions are more than just recipes; they're living repositories of cultural history. Each dish, from the ingredients chosen to the preparation methods employed, tells a story about the people who created it, their values, and their environment. Understanding these stories allows us to delve deeper into the rich tapestry of human experience, appreciating the diverse ways in which cultures have interacted with food over time.
Current Research and Clinical Trials

Current Research Directions
Current research in the field of clinical trials is focused on several key areas, including improving the efficiency and effectiveness of clinical trial design and execution. Researchers are exploring innovative methodologies to recruit and retain participants, ensuring diverse representation across demographics, and also investigating strategies for enhancing the ethical considerations in the conduct of clinical trials. These advancements aim to increase the quality and reliability of clinical trial data, ultimately leading to more effective treatments for various diseases.
A significant focus is on utilizing technology to streamline the clinical trial process. This includes the development of digital tools for patient recruitment, data collection, and remote monitoring. These technologies hold the potential to reduce costs, accelerate timelines, and improve patient engagement, thus making clinical trials more accessible and efficient.
Innovative Trial Designs
Innovative trial designs are emerging that aim to improve the speed and efficiency of drug development. These new designs often incorporate adaptive randomization strategies, allowing for adjustments to the trial as data emerges, or incorporate parallel arm designs that permit the testing of multiple treatment options simultaneously. These strategies are designed to provide more robust information about treatment efficacy earlier in the process, potentially reducing the time and resources required to bring new therapies to market.
Patient Recruitment and Retention Strategies
One of the critical challenges in clinical trials is ensuring sufficient patient enrollment. Researchers are developing sophisticated strategies for patient recruitment, including leveraging online platforms, community outreach programs, and targeted advertising campaigns. Moreover, strategies are being explored to improve patient retention throughout the trial, which includes providing clear communication, addressing patient concerns, and offering incentives.
Ethical Considerations in Trials
Ethical considerations remain paramount in clinical trials. Researchers are continually refining their protocols to ensure the safety and well-being of trial participants. This includes addressing potential biases in study design, ensuring informed consent procedures are comprehensive and understandable, and safeguarding patient privacy through data security measures. The ethical implications of using artificial intelligence and machine learning in clinical trials are also being carefully examined.
Data Analysis and Interpretation
Advances in data analysis techniques are transforming how researchers interpret results from clinical trials. Statistical methods are being refined to handle complex datasets and identify subtle treatment effects. Researchers are also exploring novel methods for analyzing data from diverse patient populations, ensuring that findings are generalizable to a broader range of individuals.
Emerging Technologies in Clinical Trials
The use of emerging technologies such as artificial intelligence, machine learning, and big data analytics is revolutionizing clinical trial operations. These technologies have the potential to enhance many aspects of the trial process, including patient recruitment, data collection, and risk stratification. AI, for instance, can be used to identify patients who are most likely to benefit from a particular treatment.
The Future of Clinical Trial Regulation
The regulatory landscape surrounding clinical trials is constantly evolving. New guidelines and regulations are being developed to address emerging challenges, such as the use of advanced technologies and the need for greater transparency in data sharing. Maintaining a balance between innovation and patient safety is a key concern. These evolving regulations aim to ensure that clinical trials are conducted ethically and efficiently, ultimately leading to the development of safe and effective treatments. The future of clinical trial regulation will be closely tied to the continued advancement of medical science and technology.
The Future of Gene Editing in Disease Prevention
Harnessing CRISPR-Cas9 for Precision Gene Editing
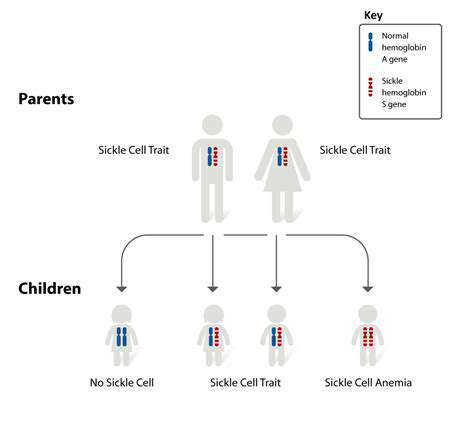
CRISPR-Cas9, a revolutionary gene-editing technology, holds immense promise for preventing genetic diseases. This system, inspired by bacterial defense mechanisms, allows scientists to precisely target and modify DNA sequences. Its ability to cut DNA at specific locations enables the correction of faulty genes responsible for inherited disorders. This precision is a significant advancement over previous gene editing techniques, minimizing the risk of unintended mutations and off-target effects. Research is actively exploring its applications in correcting mutations responsible for conditions like cystic fibrosis, sickle cell anemia, and Huntington's disease.
The potential of CRISPR-Cas9 extends beyond the correction of single gene defects. Scientists are investigating its role in modifying multiple genes simultaneously, potentially offering a more comprehensive approach to treating complex genetic disorders. This advancement could revolutionize the way we approach gene therapy, moving beyond individual gene fixes towards multifaceted solutions.
Beyond CRISPR: Exploring Alternative Gene Editing Tools
While CRISPR-Cas9 is currently the most prominent gene editing tool, researchers are actively exploring and developing alternative methods. These include base editors, prime editors, and other emerging technologies. These alternatives offer potential advantages in terms of precision, efficiency, and ease of use. By exploring a wider range of tools, scientists can potentially address limitations of current systems and pave the way for more effective and versatile gene editing solutions for disease prevention.
The development of more sophisticated and targeted gene editing tools is crucial for achieving the full potential of this field. The continuous improvement of these technologies will contribute to reducing off-target effects and improving the overall safety and efficacy of gene editing therapies.
Ethical Considerations and Societal Impacts
The advancements in gene editing technologies raise profound ethical considerations. Discussions surrounding the potential for germline editing, which affects future generations, are crucial. Careful consideration must be given to the societal implications of altering the human genome, including potential biases, equitable access, and the long-term consequences of such interventions. Public engagement and transparent dialogue are essential to navigate these complex ethical landscapes.
The ethical implications of gene editing extend beyond the scientific realm, affecting legal frameworks, societal values, and individual rights. Open dialogue and careful consideration of diverse perspectives are necessary to ensure responsible development and implementation of these powerful technologies. International collaboration and guidelines are vital to navigate the ethical challenges effectively.
The Role of Gene Therapy in Preventing Genetic Diseases
Gene therapy, along with gene editing, offers a promising avenue for preventing genetic diseases. This approach involves delivering functional copies of genes into cells to correct the genetic defect. Advances in viral vector technology, which deliver therapeutic genes to cells, are improving the safety and efficiency of gene therapy. This technique holds significant potential for treating a wide range of genetic disorders.
Gene therapy's potential extends beyond replacing faulty genes. It also encompasses techniques like gene silencing, where the expression of a problematic gene is suppressed. This approach offers a different strategy for managing genetic diseases and could be a valuable tool in the prevention toolkit.
Personalized Medicine and Gene Editing
The future of gene editing is intrinsically linked to personalized medicine. By analyzing an individual's genetic makeup, doctors can tailor treatment strategies to specific genetic predispositions. Gene editing could play a key role in this approach, allowing for the correction of disease-causing mutations identified in an individual's genome. This personalized approach promises more effective and targeted treatments, minimizing side effects and maximizing therapeutic benefits.
Ultimately, personalized medicine, coupled with gene editing, has the potential to transform healthcare. This personalized approach to disease prevention and treatment promises to be more precise and efficient, leading to better health outcomes for individuals.
Clinical Trials and Regulatory Frameworks
Extensive clinical trials are crucial to evaluate the safety and efficacy of gene editing therapies before widespread use. Rigorous testing, along with careful monitoring of patients, is essential to ensure that these therapies are not only effective but also safe. The development of regulatory frameworks is vital to ensure responsible and ethical implementation of gene editing technologies. These frameworks should balance innovation with safety and address potential risks.
Establishing clear guidelines for clinical trials and regulatory oversight is paramount. This will not only protect patients but also foster public trust in these transformative technologies. Transparency and collaboration among researchers, regulators, and the public are essential to navigating the complexities of gene editing's implementation.