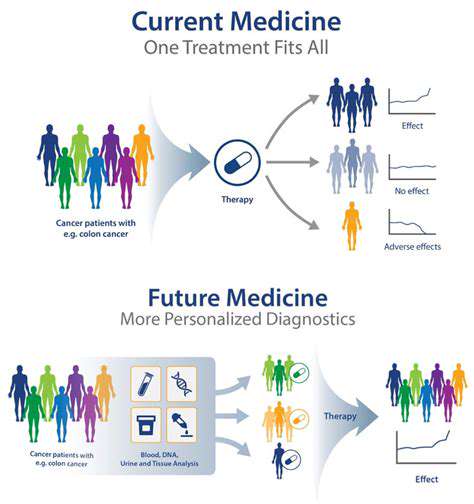
Understanding the Core Concepts
Personalized medicine represents a paradigm shift in healthcare, moving away from generalized treatments toward solutions crafted for individual biological uniqueness. Genetic differences, environmental exposures, and personal habits all contribute to how diseases develop and how therapies perform. Customizing medical interventions based on these factors enhances therapeutic success while reducing unwanted side effects. This patient-centric model evaluates not just illness pathology but also personal risk factors and biological susceptibilities.
Genetic assessment forms the cornerstone of this medical revolution. Decoding a person's DNA can uncover both disease predispositions and medication response patterns. Such insights prove invaluable for creating precise treatment protocols and preventive measures. With this genetic foresight, healthcare providers can implement early interventions that may stop diseases before symptoms appear.
The Role of Genomics in Personalized Medicine
Genomic science provides the foundation for personalized healthcare by mapping individual genetic blueprints. Through DNA analysis, medical professionals can pinpoint specific genetic variants that may elevate disease risk. These findings enable the creation of customized prevention strategies and therapeutic approaches.
Additionally, genomic research identifies genetic indicators that predict medication responses. This knowledge proves vital for optimizing treatment effectiveness while minimizing potential complications. Such precision medicine dramatically improves therapeutic outcomes compared to traditional blanket treatment approaches.
Tailoring Treatments to Individual Needs
Personalized medicine's essence lies in crafting therapies for each patient's unique circumstances. This approach considers multiple personal factors including biological age, daily habits, and overall wellness alongside disease characteristics. By accounting for these variables, clinicians can develop more potent and better-targeted treatment regimens. This individualized methodology often yields superior clinical results and enhanced quality of life.
Successful implementation requires synthesizing data from diverse sources - medical records, lifestyle information, and genetic profiles - to build a complete patient picture. This comprehensive understanding allows for more informed therapeutic decisions with higher success probabilities. Such holistic care fosters stronger doctor-patient partnerships, encouraging active patient involvement in health management.
Ethical Considerations and Future Directions
Advancing personalized medicine brings important ethical questions to the forefront. Concerns about data security, fair distribution of medical advancements, and potential algorithmic biases require careful attention. Responsible implementation and equitable access remain critical for maximizing this technology's societal benefits.
The field's future appears exceptionally promising. Emerging technologies like CRISPR gene editing and machine learning stand ready to further transform healthcare delivery. These innovations will enable even more accurate therapies, potentially improving global health outcomes dramatically.
Beyond Genetics: Integrating Lifestyle and Environmental Factors
The Role of Nutrition in Biotechnology
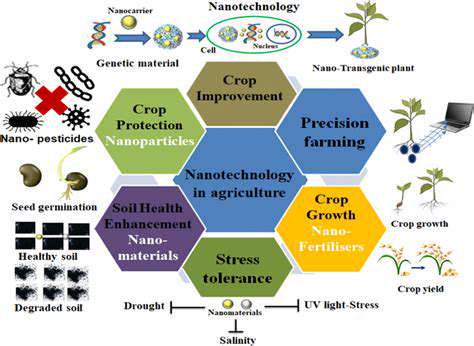
Dietary components significantly influence biotechnological applications across agriculture and human medicine. Understanding nutrient-gene interactions proves essential for optimizing biotech procedures. For instance, vitamin and mineral intake directly affects gene therapy effectiveness since these nutrients support delivery systems and cellular repair mechanisms. Moreover, customized nutrition strategies can boost agricultural biotech results by enhancing plant nutrient absorption and vitality.
Modern biotechnology increasingly acknowledges the complex relationship between diet and genetic factors. Genetic modifications designed to improve crop nutrition require complementary dietary environments to achieve full potential. Future agricultural and medical biotech developments must consider both genetic engineering and nutritional contexts to produce optimal outcomes.
Environmental Influences on Gene Expression
External conditions ranging from climate to pollution substantially impact genetic activity. This relationship proves particularly relevant for biotechnology applications involving genetically modified organisms. For example, pesticide-resistant crop genes may express differently depending on soil composition, microbial presence, and rainfall patterns. Thorough environmental understanding proves vital for creating robust biotech solutions capable of withstanding ecological variations.
Lifestyle Choices and Biotechnology Outcomes
Personal habits significantly affect biotechnological intervention success. Exercise patterns, stress management, and sleep quality all influence how bodies respond to genetic modifications or biotech treatments. Those with genetic disease risks may experience better outcomes through lifestyle adjustments that complement gene therapies. Similarly, personal habits can modify disease-related gene expression, affecting biotech treatment responses. Lifestyle factors also impact environmental biotech applications like pollution cleanup, where conditions affect engineered organism performance.
Effective biotechnology application requires considering the intricate interplay between genetics, environment, and lifestyle. This comprehensive perspective helps maximize benefits while minimizing risks, ultimately leading to more sustainable and individualized biotech solutions.
Developing Targeted Therapies: From Genes to Drugs
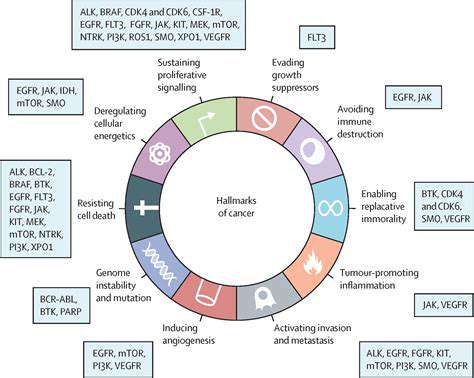
Developing Targeted Therapies: Focusing on Specific Cellular Pathways
Targeted cancer therapies mark a major therapeutic advancement. Unlike conventional chemotherapy that affects all rapidly dividing cells, these treatments precisely disrupt cancer-specific cellular processes. This selective approach minimizes damage to healthy tissue while maximizing tumor cell destruction, potentially reducing side effects and improving survival rates.
Identifying cancer's molecular drivers proves essential for target selection. By locating malfunctioning proteins, genes, or signaling pathways in cancer cells, researchers can design interventions that specifically block these abnormalities. This strategy shows tremendous potential for treating diverse cancers and enhancing treatment responses.
Exploring the Role of Genetic Mutations in Targeted Therapies
Genetic alterations often initiate and drive cancer progression. Many targeted drugs specifically inhibit proteins produced by these mutated genes, disrupting cancer-promoting pathways. Comprehensive mutation characterization enables optimal therapy selection for individual patients.
Modern DNA sequencing technologies have revealed numerous cancer-associated genetic changes. This knowledge facilitates personalized treatment planning, allowing clinicians to match patients with the most effective targeted drugs based on their genetic profiles. This precision approach is transforming cancer treatment paradigms.
Harnessing Immunotherapy for Targeted Therapy Synergy
Cancer immunotherapy, which activates the body's natural defenses against tumors, increasingly combines with targeted therapies for enhanced effects. This dual approach creates powerful treatment synergies that may produce superior clinical outcomes.
Combining these modalities provides comprehensive cancer control. Targeted treatments can improve the tumor microenvironment for immune attack, while immunotherapy boosts targeted drug effectiveness against cancer cells. This combined strategy has demonstrated improved survival in several cancer types.
The Importance of Drug Delivery Systems in Targeted Therapies
Precise medication delivery to tumor sites proves crucial for maximizing targeted therapy benefits while minimizing systemic effects. Specialized delivery mechanisms that concentrate drugs at disease sites enhance treatment effectiveness while protecting healthy tissue.
Overcoming Challenges in Targeted Therapy Development
Despite their promise, targeted therapies face development and implementation hurdles. A major challenge involves creating treatments effective against drug-resistant cancers, requiring continuous innovation to address evolving resistance mechanisms.
Long-term safety and efficacy represent another critical consideration. Understanding potential delayed effects and developing mitigation strategies remains essential for optimal patient care. Ongoing monitoring and research ensure these therapies maintain their therapeutic value across diverse populations.
The digital divide continues to present significant societal challenges, creating substantial disparities in technology access and utilization. Rural communities often experience notable disadvantages compared to urban areas regarding both internet availability and digital literacy. Addressing these gaps requires integrated solutions combining infrastructure improvements with customized education initiatives. Simply providing technology hardware proves insufficient without accompanying training for effective daily use.
Pharmacogenomics: Tailoring Drug Response to Individual Patients

Pharmacogenomics: Unveiling Personalized Medicine
Pharmacogenomics represents a dynamic discipline examining how genetic variation affects medication responses. This innovative field enables drug regimen customization for individual patients, improving treatment effectiveness while reducing adverse reactions.
By evaluating genetic profiles, pharmacogenomics identifies specific genes influencing drug processing and effectiveness. This personalized methodology holds transformative potential for modern healthcare, promising better clinical outcomes.
Personalized Drug Dosing and Efficacy
Pharmacogenomics plays a pivotal role in determining optimal medication dosages. Understanding genetic influences on drug metabolism allows clinicians to prescribe doses that achieve therapeutic benefits with minimal side effects. This precision approach significantly enhances treatment success while decreasing adverse event frequency.
Genetic variations affect drug processing speeds. This knowledge enables precise dosing that maintains therapeutic levels without exceeding safety thresholds, representing a major advance in patient care.
Minimizing Adverse Drug Reactions
Pharmacogenomics' major benefit involves predicting individual susceptibility to medication side effects. Identifying genetic risk factors helps clinicians avoid prescribing potentially harmful drugs to vulnerable patients. This preventive strategy reduces complication risks, enabling safer treatment protocols.
Improving Drug Development and Research
Beyond clinical applications, pharmacogenomics accelerates pharmaceutical innovation. Understanding genetic influences on drug response facilitates development of more effective, targeted medications. This knowledge aids in identifying promising drug candidates and optimizing existing treatments for specific patient groups.
This approach streamlines drug discovery and development. It also supports creation of more personalized treatment plans, benefiting patients across numerous conditions.
Ethical Considerations and Future Directions
Advancing pharmacogenomics raises important ethical questions about data protection and accessibility. Safeguarding sensitive genetic information remains crucial for maintaining public confidence and preventing misuse.
Future research will likely expand the catalog of drug response-related genetic markers, enabling even more precise personalized medicine. This progress will empower healthcare providers to deliver optimally tailored care for every patient.
The Future of Personalized Medicine: Ethical Considerations and Challenges
Personalized Medicine's Promise and Ethical Quandaries
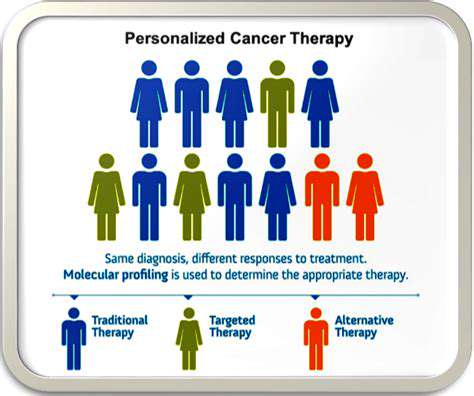
Personalized medicine's potential to customize treatments based on genetic and lifestyle factors could revolutionize healthcare. This approach enables more effective, safer therapies and improved patient outcomes. It facilitates early disease risk identification, allowing preventive measures. Understanding individual biological profiles helps predict medication responses and develop targeted treatments that maximize benefits while minimizing risks. This precision approach moves healthcare beyond standardized treatments toward truly individualized care.
However, significant ethical concerns accompany these advances. Genetic data collection raises privacy and misuse concerns. Protecting this sensitive information remains essential for maintaining public trust. Equitable access to these technologies proves equally critical to prevent worsening health disparities. Socioeconomic barriers must be addressed to ensure broad access to these medical advancements.
Data Security and Privacy in the Age of Personalized Genomics
Personalized medicine necessitates robust genetic data protection measures. Preventing unauthorized access and misuse requires advanced encryption, strict access controls, and continuous security monitoring. Data anonymization protocols help minimize risks associated with genomic information storage and sharing.
Clear policies regarding data ownership and usage must be established and communicated to patients. Informed consent processes should ensure patients understand data usage and retain control over their information. Collaboration across healthcare, research, and technology sectors can create frameworks balancing innovation with ethical considerations in genetic data handling.
Equity and Accessibility: Addressing Disparities in Personalized Medicine
Realizing personalized medicine's full potential requires addressing access and affordability challenges. Developing cost-effective diagnostic tools and therapies, along with targeted education programs, can help ensure these technologies benefit diverse populations. Addressing socioeconomic and geographic barriers prevents health disparity expansion and promotes equitable access to medical advances.
The Role of Regulation and Governance in Personalized Medicine
Clear regulatory frameworks and governance structures must guide personalized medicine development and implementation. These should address data security, privacy, and equitable access issues. Regulatory bodies need to establish ethical guidelines for genomic data use, ensuring responsible research and clinical applications. Standardized protocols for genetic information interpretation in clinical practice prove equally important. International cooperation can help ensure personalized medicine advances responsibly while upholding ethical standards.